What is ETDRS®?
Early Treatment Diabetic Retinopathy Study
ETDRS® stands for Early Treatment of Diabetic Retinopathy Study. The first ETDRS® trial was a multisite, randomized clinical trial designed to evaluate argon laser photocoagulation and aspirin treatment in the management of patients with early proliferative diabetic retinopathy. A total of 3,711 patients were recruited to be followed for a minimum of 4 years to provide long-term information on the risks and benefits of the treatments under study. Foregoing the traditional Snellen optotypes, which were at that time considered standard, better charts were needed for more accurate results. The ETDRS® study introduced three new visual acuity charts, the “Original Series” with follow-up studies on those charts resulting in an additional “2000 Revised Series” which have a different letter arrangement for more homogeneous results. Both the original and revised series charts use Sloan letters. A variety of other optotypes are accepted worldwide. ETDRS® equipment and testing have evolved into what is considered the gold standard of current-day clinical trials involving vision testing.
Historical Timeline
1971
In 1971, shortly after the National Eye Institute (NEI) establishment in 1969, NEI supported several clinical trials that helped find effective treatments for diabetic retinopathy, including the Diabetic Retinopathy Study (DRS), The Diabetic Retinopathy Vitrectomy Study (DRVS), and eventually the Early Treatment Diabetic Retinopathy Study (ETDRS). 1
1976
By April of 1976 the results from the Diabetic Retinopathy Study proved that laser treatment is effective for treating diabetic retinopathy.
1979
In December of 1979 the first clinical trial began for the Early Treatment of Diabetic Retinopathy Study. This study’s patient eligibility criteria were open to men and women between the ages of 18 and 70 years with moderate or severe non-proliferative diabetic retinopathy or mild proliferative retinopathy in both eyes. The eligible patients had no previous photocoagulation treatment and with visual acuity of 20/40 or better (20/200 or better if macular edema is present). Precision Vision was proudly there for this first ETDRS® clinical trial and honored to be supporting and supplying the same trusted equipment utilized in today’s clinical trials.
1989
By October 1989 the results from the Early Treatment Diabetic Retinopathy Study provided further evidence that laser treatment is highly effective in treating diabetic retinopathy. These critical results lead to the future of gold standard vision testing equipment and subsequent research on diagnosing and treating diabetic retinopathy earlier.
What are ETDRS® Charts?
To understand what an ETDRS® chart is, we should first discuss why the ETDRS® charts were designed.
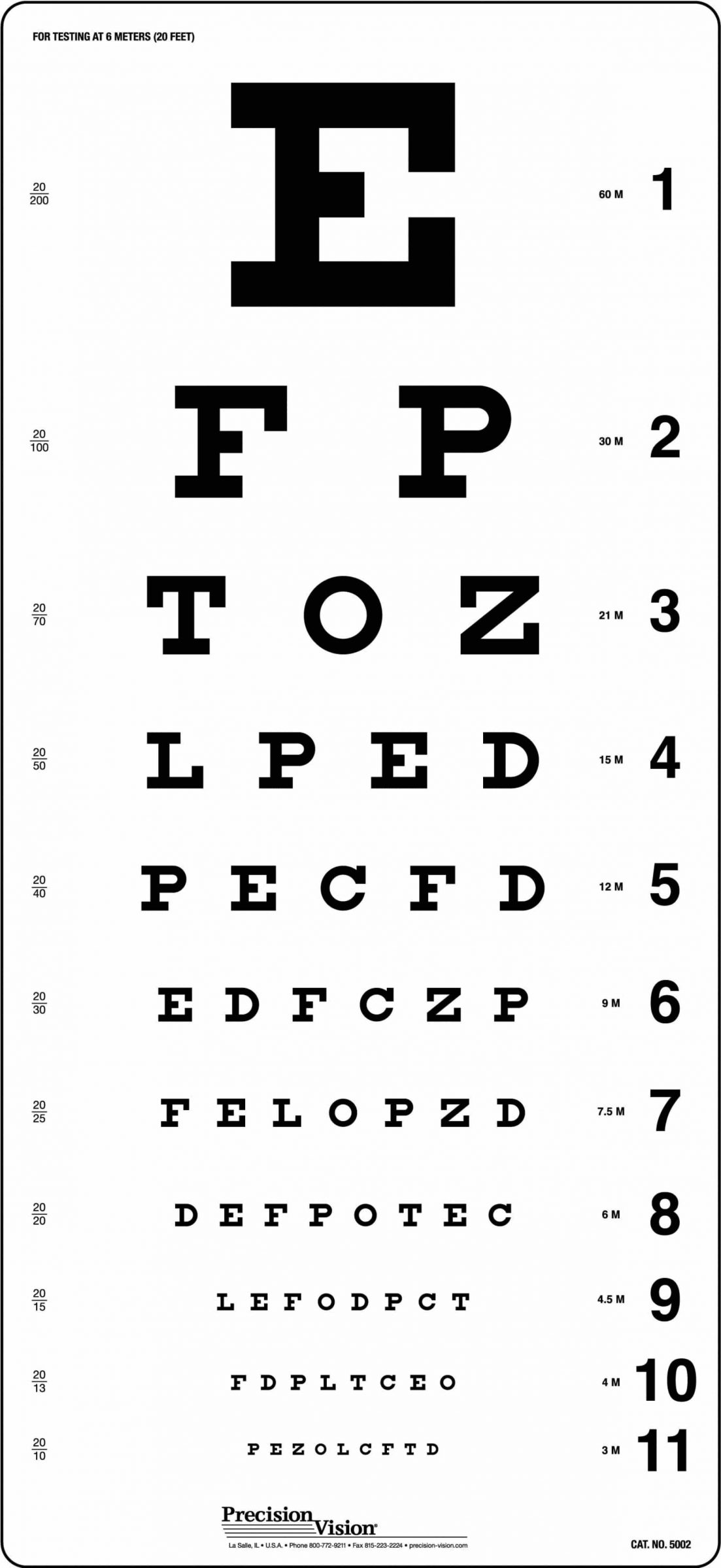
Prior to the creation of ETDRS® charts, many commonly used visual acuity charts had a different number of letters on each line like the chart shown here.
Charts similar to this one traditionally allowed one mistake per line, therefore, having a different meaning at different levels of visual acuity. To have a visual acuity of 6/6 (20/20) with this chart, one must correctly identify seven of eight letters (89%) on that line, but a visual acuity of 6/30 (20/100) requires the correct identification of only one of two letters (50%). This example also uses serif fonts and a letter selection which are not used for clinical trials.
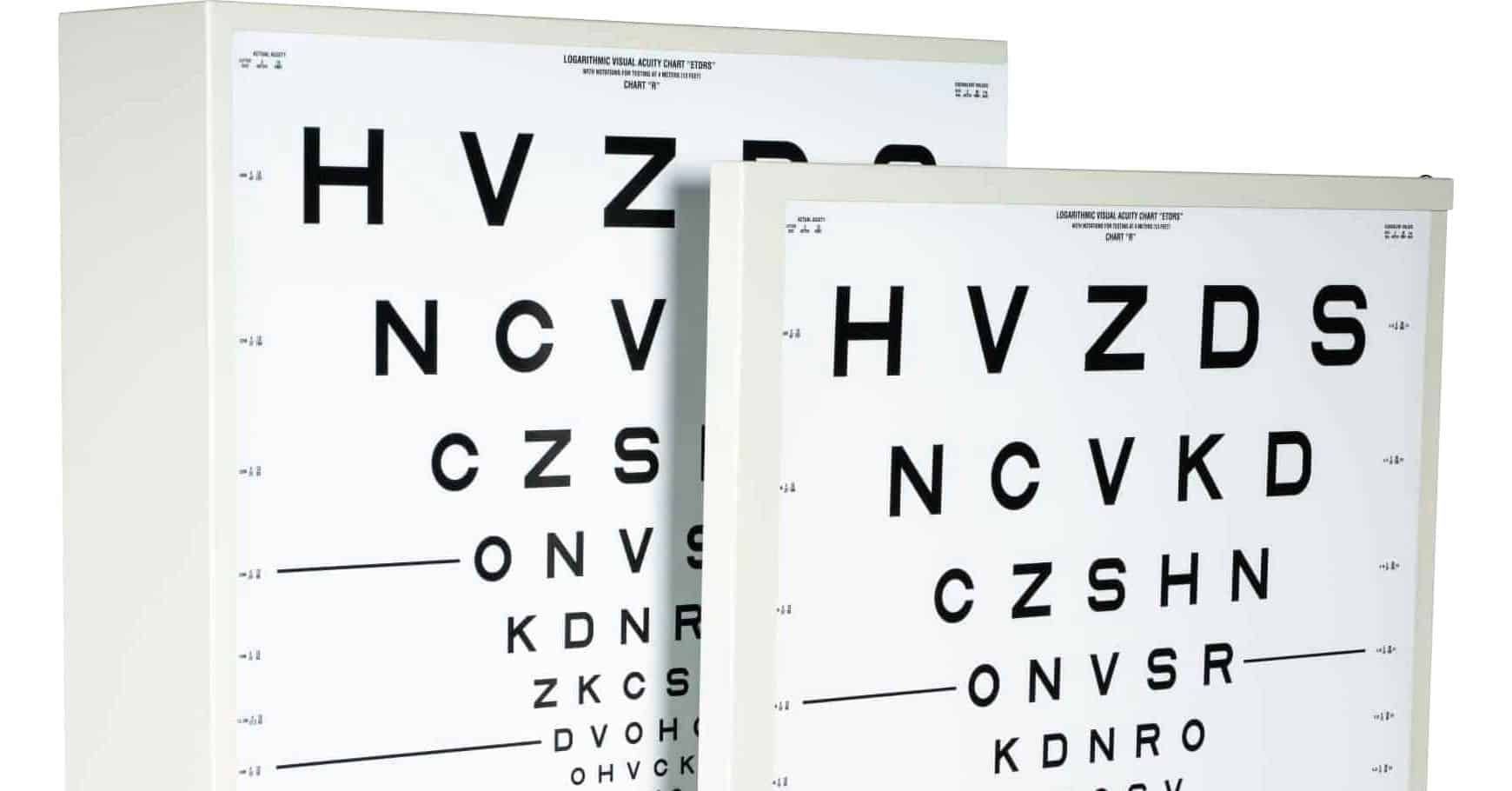
ETDRS® charts have five Sloan letters on each line; the lines are of equal difficulty, and there is a geometric progression (LogMar) in letter size from line to line. LogMar stands for Log of Minimum Angle of Resolution. The design of this chart allows for a logarithmic or proportional change in letter size and spacing. This results in the visual angle on the chart doubling every three lines. In addition, by design the spacing between rows is logarithmic following the size of the letters. Spacing between letters is also uniform and correlates to the size of the letter. This provides a similar task for each line on the chart with the letter size being the only variable. Charts with different letter sequences are used for testing right and left eyes, with a third chart often used for refractions.
The ten Sloan letters chosen for the ETDRS® charts were assigned a difficulty score based on how often the letter is read correctly at the visual acuity threshold. These letter difficulties are approximately equal to a Landolt Ring (Landolt C). The letters in each line were arranged so that no words or acronyms were spelled out.
The ETDRS® charts are 64.8 cm wide and 62.2 cm high (25.5 x 24.5 inches). High contrast optotypes are printed onto non-reflective, white polystyrene which is trans-illuminated (or retro-illuminated) within a properly calibrated illuminator cabinet. The illuminator cabinet produces uniform, standardized illumination across all testing sites. 4

How do you test and score ETDRS® Charts?
To begin, testing should be done with Best Corrected Visual Acuity ( BCVA ) patients should be either seated or standing 4 meters from the chart. Beginning with Chart 1, the right eye is tested with the left eye occluded. Following the completion of testing the right eye, the left eye is tested with Chart 2 while covering the right eye.
Reading slowly, each letter is scored as right or wrong. Correct letters are circled on the scoresheet. Each letter read correctly is assigned a score and each line is totaled at the end of testing. Typically start/stop rules are well defined within trial protocol.
For subjects with reduced vision at 4 meter, testing distance should be reduced to 1 meter. Testing protocol will define rules for testing at 1 meter versus 4 meters.
Scoring in ETDRS® is typically done by letter count. Visual Acuity Score ( VAS ) awards one point for every letter correctly guessed. Whereas LogMar reduces the score by .02 for each letter correctly guessed. For example; when a patient correctly reads all lines, including the five letters on the 20/20 line, the VAS score is 100 points and LogMar score is 0.0.
Additional testing and scoring details are available by request.
Measurements of visual acuity using these charts provide reproducible visual acuity information in a format that facilitates quantitative data analysis across multiple sites, countries, and subjects.
What is an ETDRS® Illuminator Cabinet
Illumination standards for visual acuity testing were set by the first NEI clinical trial. There are a few options and accessories for ETDRS® illuminator cabinets. The traditional retro-illuminated cabinet is nearly the same cabinet utilized for over forty years, and has been manufactured by Precision Vision, Inc since the very first trials. Over the last decade an LED ETDRS® illuminator cabinet has been added to their clinical trial and research line up. The LED option allows not only the traditional illumination requirements but allows easy access to multiple other illumination values.
Both ETDRS® cabinet options offer storage for charts in the back for quick access and for mobility, a five – leg caster base is available.
Additional details are available upon request. We are driven to make a difference! Help us help you! Request more information on ETDRS® and clinical trials today!
References:
- National Eye Institute (NEI)-53, Early Treatment Diabetic Retinopathy Study (ETDRS)
- Kinyoun J, Barton F, Fisher M, Hubbard L, Aiello L, Ferris F 3rd. Detection of Macular Edema. Ophthalmoscopy Versus Photography Early Treatment Diabetic Retinopathy Study Report Number 5. The ETDRS Research Group. Ophthalmology. 1989 Jun
- American Journal of Ophthalmology, Vol 116, No. 6, December 15, 1993; Frederick L. Ferris III, M.D., Aaron Kassoff, M.D., Sylvan B. Green, M.D., and Roy C. Milton, PhD.
- American Journal of Ophthalmology, Vol. 94:91-96, No. 1, July 1982; Frederick L. Ferris III, M.D., Aaron Kassoff, M.D., George H. Bresnick, M.D., Ian Bailey, M.D.
- Letter-Count Scores, August Colenbrander, MD – San Francisco
- Ferris, F., and Sperduto, R.: standardized illumination or visual acuity testing in clinical research. Am. J. Ophthalmol. 94:97, 1982.





